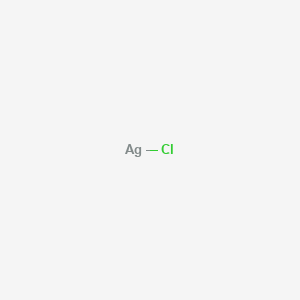
Silver Chloride CAS 7783-90-6


Factory wholesale Silver Chloride CAS 7783-90-6
- Appearance:Liquid
- Purity:99.8%
- Delivery:30days
- Sample Available:Available
- Payment:L/C,T/T,D/P,Paypal,Money Gram,Western Union
- Incoterm: FOB,CFR,CIF,EXW,FCA,CPT,CIP
- Transporta:Ocean, Land,Air, DHL,TNT FedEx
Name: Silver Chloride
CAS: 7783-90-6
MOQ: 1KG
Directory Guidance on Silver Chloride
Chemical Structure
Basic Info:
| Melting Point | 455 °C (lit.) |
| Boiling Point | 1550 °C |
| Density | 5.56 |
| Vapor Pressure | 1 mm Hg ( 912 °C) |
| Refractive Index | 2.071 |
Silver Chloride Introduction:
Silver Chloride(AgCl), This simple inorganic compound formed by the combination of silver and chlorine elements has a concise and clear chemical formula, but plays a crucial role in science and industry that far exceeds its molecular complexity. Its most well-known physical state is a white or off white crystalline powder, with a delicate texture and typical ionic crystal characteristics.
The newly prepared Silver Chloride powder usually presents a pure white color, but it is extremely sensitive to light and undergoes rapid photochemical reactions when exposed to light, resulting in a gradual darkening of the color and ultimately presenting a grayish purple or even black color. This significant photochromic effect is one of its most important chemical behaviors and the core foundation of its historic applications.
From a chemical analysis, the core characteristic of Silver Chloride is its extremely low water solubility. At room temperature (25 ° C), its solubility in water is only about 0.0019 grams per liter, which is negligible when converted to molar concentration. This characteristic makes it a crucial precipitate in qualitative and quantitative analytical chemistry. This precipitation behavior has high specificity and sensitivity, and is the classic method basis for detecting and quantifying chloride ions or silver ions.
In addition, although Silver Chloride is difficult to dissolve in pure water, it exhibits certain solubility in certain specific solvents or solutions. It can significantly dissolve in concentrated hydrochloric acid, ammonia water, potassium (sodium) cyanide solution, and sodium thiosulfate solution, forming soluble complexes. This chelation dissolution behavior has important application value in analytical chemical separation, electroplating processes, and photographic chemistry. Meanwhile, it can conduct current in a molten state or at high temperatures, making it a typical ion conductor, which is related to the mobility of ions in its crystal structure.
Controlling the concentration, mixing rate, and precipitation aging conditions of reactants can to some extent regulate the size, morphology, and purity of the obtained Silver Chloride particles, which is crucial for their subsequent application performance. In history, its discovery and application can be traced back to the ancient alchemy period, but the systematic scientific research and widespread application of it began with the development of chemistry in the 18th and 19th centuries. In the early days, it was mainly used for the identification and separation of silver, and the discovery of its photosensitive properties completely changed the historical process of image recording.
Nature and Specifications:
| Item | Specification |
| Product Name | Silver Chloride |
| CAS No. | 7783-90-6 |
| Appearance | Powder |
| Shelf Life | 2 years |
| Packing | As Your Requirements |
| Solubility | 0.00188g/l |
| Form | beads |
| Color | Yellow |
| Specific Gravity | 5.56 |
Product service:
- Certificate Of Analysis (COA)
- Material Safety Data Sheet (MSDS)
- Route of synthesis (ROS)
- Method of Aanlysis (MOA)
- Nuclear Magnetic Resonance (NMR)
- Packing pictures and loading video before loading
- Free Sample
- Factory audit
The Application Situation Of Silver Chloride
The properties of Silver Chloride have established its status as a benchmark substance for analytical chemistry. The specificity of its precipitation reaction, the typicality of its precipitation morphology, and the accuracy of its solubility product data make it the “gold standard” for quantifying chlorine or silver by gravimetric analysis, and an important reference for verifying the accuracy of other analytical methods. At the same time, it is a precipitate that appears as an indicator of the end point in complexometric titration (such as the Mohr method and Fayon method for determining chloride ions), and its sharp generation or dissolution indicates the completion of the titration reaction.
In the field of instrumental analysis, ion-selective electrodes based on Silver Chloride are commonly used sensors for determining the activity of chloride ions in solutions. Its core sensitive membrane is made of the product or a mixture of the product and silver sulfide, and uses the membrane potential to respond to the chloride ion concentration to achieve rapid and online detection. It is widely used in environmental monitoring, biological fluid analysis, industrial process control and other fields. Its stability and relatively easy preparation characteristics also make it a common substance used to calibrate instruments or as a matrix material in spectral analysis (such as X-ray diffraction).
The invention and development of photography is undoubtedly the most brilliant application chapter of Silver Chloride. The core photosensitive substances of traditional silver halide photographic materials are Silver Chloride, silver bromide (AgBr) and silver iodide (AgI), which are uniformly dispersed in the gelatin matrix in the form of microcrystals and coated on the film base (film or photographic paper) to form a photosensitive emulsion layer.
Silver Chloride emulsion is mainly used in low-speed, high-contrast photographic paper (such as printing paper and enlargement paper) because it is sensitive to blue-violet light and has a relatively slow development speed. It can produce black-and-white photos with pure tones and hard tones, meeting the needs of specific artistic creation and archival records. Even today when digital imaging technology is highly developed, traditional photographic materials based on this product still have a place in artistic photography and professional fields due to their unique texture and craftsmanship.
In the field of electronics and materials science, Silver Chloride has found new application directions with its ionic conductivity and unique photoelectric properties. As a typical fast ion conductor (especially at high temperature or doped state), this product and its composite materials are studied for solid electrolytes, especially in miniaturized solid-state batteries, electrochemical sensors and ion switching devices. Its relatively high silver ion mobility is the key to achieving fast ion transport.
In optoelectronics, Silver Chloride is an important photosensitive semiconductor material. Its bandgap is about 3.25 eV, and it mainly absorbs ultraviolet light. Taking advantage of its photogenerated carrier characteristics, it is explored for use in ultraviolet detectors and certain special photocatalytic reactions. The heterojunction composite material formed by loading Silver Chloride nanoparticles onto the surface of other semiconductors (such as titanium dioxide TiO₂) can effectively promote the separation of photogenerated electron-hole pairs, significantly improve the activity of the material in photocatalytic degradation of pollutants or photolysis of water to produce hydrogen under visible light irradiation, and is one of the hot spots in environmental governance and new energy material research.
The Advantages Of Silver Chloride
The core advantage of Silver Chloride is first reflected in its excellent chemical stability. As a typical ionic crystal, Silver Chloride exhibits extremely high chemical inertness in a dry and light-proof environment under normal temperature and pressure.
It is insoluble in water, dilute acids, dilute alkalis and the vast majority of organic solvents. It is also quite stable to components such as oxygen and carbon dioxide in the atmosphere and is not prone to oxidation-reduction or decomposition reactions. This inherent stability ensures its long-term reliability as an analytical reference substance or electrode material and the repeatability of the data.
Its precipitation reaction is highly specific: under appropriate acidity conditions, silver ions almost only react with chloride ions to form typical white curd-like precipitates, and do not produce interfering precipitates with other common anions (such as nitrate, sulfate, acetate, etc.).
This specificity makes the Silver Chloride precipitation method one of the most authoritative and reliable methods for qualitatively detecting chloride ions and quantitatively determining the content of chlorine or silver, and its results are often used as the basis for arbitration. Even in complex sample matrices, the accurate determination of chloride ions can be achieved through simple masking or separation steps, which is an advantage that many other detection methods find hard to match.
From the perspective of material preparation and processing, Silver Chloride also has significant advantages. Its synthesis process is mature, simple and easy to scale up. The product can be obtained efficiently and in high yield through the precipitation reaction of silver nitrate with sodium chloride or hydrochloric acid.
By controlling reaction conditions (such as concentration, temperature, mixing rate, and aging time) and subsequent treatments (washing and drying), it is relatively easy to regulate the physical form of the product, such as particle size, distribution, specific surface area, and crystal form, to meet the specific requirements of different application scenarios for the physical properties of materials.
Furthermore, Silver Chloride has good fluidity after melting and can be prepared into relatively dense bulk materials with certain mechanical strength and optical uniformity through hot-pressing molding or melt casting processes. This is crucial for its applications in fields such as infrared optical Windows.
Silver Chloride also shows favorable characteristics in terms of the environment and safety. Its own toxicity is relatively low, mainly based on the low mobility and low bioavailability of silver ions in organisms. Although silver ions have antibacterial properties, their solubility in water is extremely low, which limits the release rate and total amount of silver ions. Therefore, the environmental risk is relatively controllable.
The disposal of Silver Chloride after its disposal is relatively simple. Metallic silver, as a valuable precious metal, can be effectively recycled and reused from waste materials containing this product (such as expired photosensitive materials, electronic waste). Usually, it is reduced and purified after dissolution (such as cyanide method, thiosulfate method or acid oxidation method), achieving the recycling of resources and conforming to the concept of sustainable development. This recyclability not only reduces the cost of long-term use, but also alleviates the environmental burden.
Contact Us
Product Package picture:



Related References:
Chemicalbook-Silver Chloride
Silver Chloride Manufacturer
Contact Us
As an experienced Silver Chloride manufacturer and supplier, Look Chemical is committed to producing and selling high quality products.
We cooperate and trade with 6000+ factories around the world, and our high-quality products and excellent services make us enjoy a high reputation internationally.
As Silver Chloride CAS 7783-90-6 supplier, Look Chemical provides supply chain solutions to partners and customers in a wide range of industries. We offer competitive pricing and quality products.
If you have a demand for this product, please contact our company’s sales staff, we will provide you with a solution in the shortest time.
Transport proposal

1. For products ≤50kg, we recommend using express delivery, which is usually called DDU service (discounted, convenient).
2. For products ≤500kg, we generally recommend air freight, which is usually called FOB, CFR or CIF service (fast and efficient).
3. For products >500kg, we generally recommend shipping by sea, which is usually called FOB, CFR or CIF service (economical, safe).
4. For high-value products, please choose air or express to ensure the safety of product transportation.
Shandong Lookchemical service:
* Timely reply and 24 hours online, the professional team will provide you with the most favorable prices and high-quality products.
* The sample supports testing and inspection.
* Each batch of products will be tested to ensure that its quality meets user needs.
*Packaging can also be made according to customer requirements.
*Any inquiries will be answered by our relevant personnel within 24 hours.
*We will provide you with commercial invoice, packing list, packing list, COA, health certificate and certificate of origin if you need it. If your market has other special requirements, please let us know.
*We will monitor the logistics information in real time and will share the information with you.
* You can consult us at any time if you have any questions about the product, and we will answer you in time.
*If you have any questions about the product, you can report it to us, we will deal with it in time for you, and the product can be returned.
Contact Us
Frequently Asked Questions(FAQ):
We will make samples before mass production, and after sample approved, we’ll begin mass production. Doing 100% inspection during production, then do random inspection before packing.
Our MOQ is 1kg. But usually we accept less quantity such as 100g on the condition that sample charge is 100% paid.
Yes. We’ll give you product analysis report before shipping.
Different quantity has different discount.
Yes. Welcome to visit.
You can get free samples for some products,you only need to pay the shipping cost or arrange a courier to us and take the samples. You can send us your product specifications and requests,we will manufacture the products according to your requests.