What are Plasticizer?

introduction:
Plasticizer, a key additive that imparts or enhances plasticity and flexibility in polymers, has been deeply applied to all core aspects of the modern industrial system. According to the latest annual assessment data from Statista, an industry authority, the global plasticizer market value has climbed to an astonishing $14.5 billion in 2023, and is expected to steadily expand to $17.6 billion in 2028.
The huge market base reveals the irreplaceable and important position of this type of chemical in many pillar industries of the national economy – plastic products, medical devices, food packaging, electronic products, automotive industry, construction materials, etc. Almost any production link involving polymer materials cannot be separated from the fine control of plasticizer.
Table of Contents
- introduction
- 1.The Historical Evolution and Basic Concepts of Plasticizer
- 2.The Plasticizer Plasticizing Mechanism and Core Indicators
- 3. Analysis of Mainstream Plasticizer Types and Their Key Characteristics
- 4.Health and Ecological Concerns Caused By Plasticizer Exposure
- 5.The Development of Environmentally Friendly Plasticizers
1.The Historical Evolution and Basic Concepts of Plasticizer
Humans have a long history of using natural substances to improve the flexibility of materials. The invention of nitrocellulose (also known as celluloid) in the mid-to-late 19th century marked the beginning of the application of modern synthetic polymers. However, nitrocellulose is naturally brittle and flammable, and its processing and practical limitations are obvious.
A breakthrough attempt in 1865 introduced camphor as its plasticizer, creating the first commercially significant artificial plastic. Camphor effectively inserts between nitrocellulose chains due to its molecular compatibility, significantly weakening the interchain forces, giving celluloid the necessary flexibility and plastic processing properties, allowing it to be widely used in billiards, film and other fields. This is the starting point of modern plasticizer technology.
The real take-off of the modern plastic industry began with the commercialization of polyvinyl chloride (PVC) and the popularization of phthalate plasticizers. In its original state without the addition of plasticizer, PVC is a hard and brittle polymer with a glass transition temperature (Tg) of more than 80°C. At room temperature, it is like a hard sheet, and its application range is extremely limited.
In the early 1930s, the successful development and large-scale production of efficient and low-cost dioctyl phthalate (DOP, also known as DEHP) completely changed the fate of PVC materials. By adding a large amount (usually up to 30%-50% of the resin weight), DEHP and other phthalate plasticizers can penetrate into the PVC polymer chains, effectively increase the distance between molecular chains through physical forces (mainly van der Waals forces), significantly reduce the barrier of chain segment movement, and thus reduce the Tg of PVC to below room temperature, turning it into a soft, flexible and elastic material.
With the rapid development of modern chemical technology, the functional role of plasticizer is also constantly expanding and deepening. Modern high-performance plasticizer systems have expanded from simply improving flexibility to giving polymers improved processing performance (such as reducing melt viscosity and inhibiting melt fracture), enhanced low-temperature toughness, flame retardant synergy, optimized light and heat stability, and special environmental tolerance (oil resistance, solvent resistance, extraction resistance) and other key properties to meet the increasingly complex and stringent performance requirements of end products.
Taking the PVC dashboard skin of automotive interiors as an example, the design must simultaneously meet multiple physical and chemical property requirements such as low-temperature toughness (no brittle cracking at -30°C), UV aging resistance (no yellowing or cracking under long-term sunlight exposure), flame retardancy (in compliance with vehicle safety standards), resistance to volatile organic solvents (such as detergents), and processing fluidity that adapts to complex surface hot pressing molding.
The formulation design of this type of material is highly complex, often requiring the coordinated compounding of 2-3 main plasticizers (such as phthalates and trimellitates), supplemented by epoxy auxiliary plasticizers to improve thermal oxygen stability, and adding flame retardants, stabilizers and other additives to achieve comprehensive goals. A single-component plasticizer is difficult to cover such multi-dimensional performance requirements.
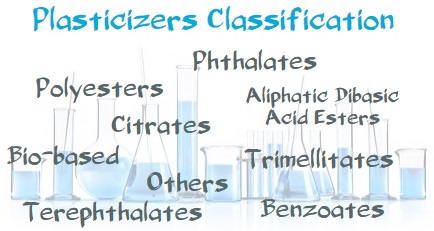
2.The Plasticizer Plasticizing Mechanism and Core Performance Indicators
The core mechanism of the effectiveness of Plasticizer lies in its swelling, dilution and isolation between molecular chains with polymer resin. When the Plasticizer molecules are fully dispersed in the polymer matrix, they essentially play a role similar to “molecular lubricant”: the considerable volume of Plasticizer small molecules intersperse and embed between adjacent polymer molecular chains, strongly weakening or shielding the cohesive force between segments (especially non-polar or weakly polar polymers) – mainly including van der Waals force and dipole interaction.
Macroscopically, this process is manifested in a significant increase in the free volume of the polymer, a sharp increase in the activity of the chain segments, and a significant shortening of the relaxation time (time required for movement) of the chain segments. The decrease in the glass transition temperature Tg indicates a decrease in the temperature required for the polymer to enter the highly elastic state from the glassy state, which is intuitively manifested as the material showing excellent flexibility and ductility at a lower temperature.
Experimental tests show that adding 50% by weight of DOP to PVC can sharply reduce the material Tg from about 85°C (pure resin) to below -30°C, with a very significant effect. On a microscopic scale, there is no substantial chemical bonding between the plasticizer and the polymer chain; their connection mainly relies on physical forces, which poses a potential risk for subsequent migration and precipitation.
The Core Indicators of Plasticizer
The key indicators for judging the performance of plasticizers are diverse and highly dependent:
1. Compatibility: This is the basic premise for the plasticizer to work. Excellent plasticizers should form a uniform, stable, and long-term coexistent miscible state with the resin matrix at the molecular scale, and never have macroscopic phase separation or “sweating” (oil seepage).
2. Efficiency: It is usually measured by the minimum amount of plasticizer required to achieve a specific hardness (such as Shore A hardness) or target modulus. High-efficiency plasticizers can achieve the ideal flexibility of the material with less addition.
3. Migration & Extraction Resistance: This property determines the service life and safety risk level of the material.
4. Permanence: It covers volatile loss, solvent extraction detachment, and exudation under stress. High-boiling point, high-molecular-weight plasticizers have natural volatility inertness. Those with stronger intermolecular forces (such as hydrogen bonds or highly polar groups) are also not easily extracted by non-polar solvents.
5. Processing & Rheology: Excellent plasticizers can significantly reduce the viscosity of the resin melt, improve fluidity, shorten the mixing and plasticizing time, and improve the efficiency of extrusion or injection molding. Phthalates usually have both good plasticizing effect and balanced fluidity. Improper selection will lead to problems such as processing difficulties, material deposition and scorching on the wall of the equipment, or screw torque overload.
3.Analysis of Mainstream Plasticizer Types and Their Key Characteristics
1. Phthalate plasticizers:
They have long occupied more than 75% of the global plasticizer consumption (the core position is difficult to shake. They have the advantages of outstanding cost-effectiveness (abundant raw materials, mature synthesis process), wide compatibility, good processing lubrication performance, high efficiency, and balanced comprehensive performance. Typical varieties include: diisononyl phthalate (DINP), diisodecyl phthalate (DIDP), di(2-propylheptyl) phthalate (DPHP). Di(2-ethylhexyl) phthalate (DEHP or DOP)
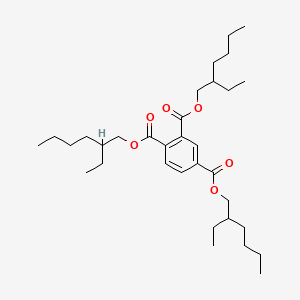
2. Trimellitate plasticizers (TOTM, TINTM):
With higher thermal stability and durability, the initial decomposition temperature of tri(2-ethylhexyl) trimellitate (TOTM) is greater than 300°C (far exceeding the 230°C of phthalates), and it has excellent migration resistance (not easily extracted by soap or detergent), and is particularly suitable for automotive cable sheaths (temperature resistance above 105°C), aviation wires, and sealing products for extreme high temperature conditions. However, its cost is significantly higher than that of phthalates, which is its shortcoming.
3. Epoxy Plasticizer:
Epoxy vegetable oil: especially epoxidized soybean oil (ESBO), is environmentally friendly and non-toxic A benchmark representative of plasticizers, with moderate to good compatibility with PVC. Widely used in food contact materials that require high safety, children’s toys (in compliance with mandatory toy safety standards in many European and American countries such as EN71), medical hoses, etc.
Epoxy fatty acid monoesters (such as epoxy tetrahydrophthalate dioctyl ester): The performance is between epoxy oil and phthalate esters, with good compatibility and improved thermal stability, especially in wire and cable materials.

4. Bio-based and renewable Plasticizer:
Citrate esters: derived from natural citric acid, safe and non-toxic (FDA compliant 21CFR standard), with good compatibility and moderate plasticizing efficiency, widely used in high safety demand fields such as pharmaceutical packaging (tablet sustained-release coating), cosmetic packaging, and children’s toys.
Vegetable oil-based polyol esters: Chemical modification (such as epoxidation and transesterification) of natural oil esters such as castor oil improves compatibility with PVC, heat resistance and mechanical properties. The performance of some varieties is close to that of dioctyl phthalate (DOP), and the molecular design is flexible, becoming the new favorite of the industry.
5. Polyester Plasticizer:
Mostly condensed by diacids (adipic acid, sebacic acid) and diols (propylene glycol, 1,2-butanediol), with a molecular weight of 1800-6000 Da varies. The biggest highlight is the extremely excellent durability: ultra-low volatility, ultra-low migration and super oil and solvent extraction resistance (because its molecular chain is partially entangled with the PVC chain or forms a network effect).

6. Other functional/special plasticizers:
Phosphate esters (such as triphenyl phosphate TPP, tritolyl phosphate TCP): have excellent flame retardancy (such as TCP limiting oxygen index can reach 35%, significantly inhibiting the spread of flames), usually used with conventional plasticizers to enhance the overall flame retardancy of PVC.
Benzoic acid Esters (such as diethylene glycol dibenzoate DEDB, dimethyl nylon): The plasticization rate is extremely fast, which can significantly reduce the processing temperature and equipment energy consumption, and has unique advantages in the surface drying layer of PVC foam floor leather and artificial leather (inhibiting sticking to the roller).
Adipates (such as Dimethyl azelate), sebacates (such as dioctyl sebacate DOS): The king of outstanding low-temperature resistance (can make PVC resistant to low temperatures of -50°C to -60°C), used for cables, films, special seals used in cold areas, and even material components in satellite low-temperature environments.
4.Health and Ecological Concerns Caused By Plasticizer Exposure
The core of the current plasticizer safety controversy focuses on specific phthalates (such as low molecular weight ones such as DEHP, DBP, BBP, DIBP, etc.). A large number of cell experiments, animal models and epidemiological surveys around the world strongly suggest that they have endocrine disrupting effects. The European Chemicals Agency ECHA and the US National Toxicology Program NTP have evaluated DEHP, DBP, etc. as Class 1B or equivalent hazard level substances with reproductive toxicity.
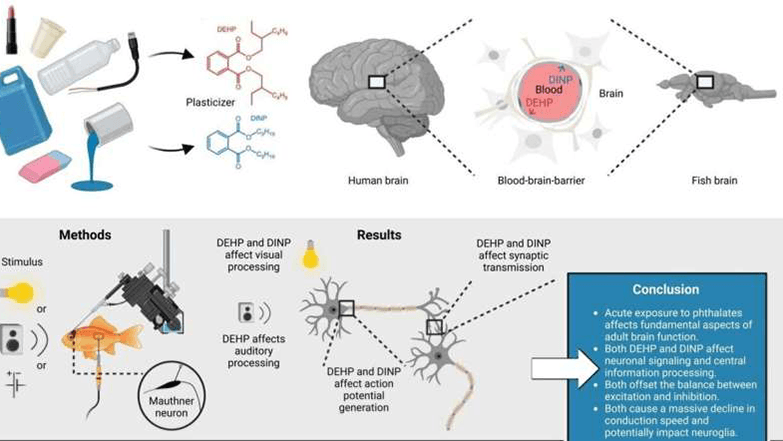
The important medium for plasticizer to enter the biosphere is its continuous release behavior from the final product. Experimental simulation data show that after an ordinary PVC bathroom waterproof mat is operated for 1000 hours under continuous watering, heating (40°C) and trampling (simulating physical wear), the concentration of DINP on its surface can drop by more than 15%, part of which is emitted into the indoor air in a volatile form, and part of which enters the environmental water body with the flushing water.
In the environmental medium, plasticizer shows significant environmental persistence. Its lipid solubility makes it easy to adsorb on suspended particles in water, riverbed mud, sludge and soil organic matter. Especially in aquatic sediments, its half-life can reach months or even years. More seriously, it is widely detected in biological samples at various levels of the food chain, showing a clear trend of bioaccumulation and amplification along the food web.
5.The Development of Environmentally Friendly Plasticizers
1. The migration from basic phthalates to specialty/environmentally friendly products has become a trend: According to industry insights released by Grand View Research, the global non-phthalate plasticizer market is expected to continue to grow at a compound annual growth rate of 6.5% (forecast period 2023-2030). The focus of industrial investment is clearly biased towards the development and capacity expansion of environmentally friendly specialty products
2.Innovative design of molecular structure is the key technical path to break through the core bottleneck: The focus of solving the efficiency (such as insufficient plasticization requiring higher addition), imbalance of high and low temperature performance (such as brittleness at low temperature), compatibility limitations (poor formulation compatibility) and cost disadvantages of traditional plasticizer substitutes lies in molecular engineering:
Targeting efficiency bottlenecks: Develop designs with multiple strong polar groups (such as increasing ester group density, introducing cyclic structures such as pyridine rings) while optimizing molecular chain length and branching degree.
Improve thermal and migration stability:
Rigid groups with higher thermal stability can be introduced into the molecular skeleton, or plasticizers with reactive groups can be synthesized to partially chemically bond with the polymer chain in the final product, thereby greatly locking the molecule and curbing migration escape;
Advanced application of bio-based raw materials: Using 12-hydroxystearic acid, a unique component in castor oil, as a key intermediate, a castor oil-based polyester plasticizer with good low-temperature toughness and excellent extraction resistance was synthesized, and its performance data is close to diisononyl phthalate (DINP) in some key indicators.
3. Strengthening regulatory compliance and life cycle management has become a necessary capability for corporate survival: from raw material traceability, green processes in chemical synthesis to finished product testing compliance, waste product recycling cycle path design and other processes, a complete environmental compliance chain is formed. Enterprises need to invest resources to build a product life cycle management system that runs through “molecular design → compliant production → risk assessment → usage tracking → recycling and regeneration” to meet the threshold requirements of the international market for “green chemicals”.
4. Exploratory interdisciplinary technologies bring disruptive possibilities: for example, using gene-edited microbial fermentation pathways to produce platform chemicals with specific chain lengths and functional groups; using nanoparticle modification or in situ polymerization of plasticizer prepolymers to improve their interface bonding and dispersion effects with resins, and designing medical elastomers that are highly matched to the mechanical behavior of human soft tissues (replacing the traditional PVC + DEHP combination). For example, technical alternatives based on silicone hydrogels, polyurethane hydrophilic materials and other plasticizer-free solutions in extracorporeal circulation tubing and infusion devices are gradually expanding their clinical application range.

